Introduction:
Textural properties are characteristics that refer to the porous structure to the catalyst porous structure and play a crucial role in its performance. In an FCC catalyst, these properties derive mainly from the zeolite – a crystalline solid the tridimensional network of which imparts it channels and cavities, and from the matrix – a non-crystalline structure which contributes directly and indirectly to the textural properties.
These features are directly linked to the capacity of interaction with reagents, including selectivity, availability of active sites and chemical species ease of diffusion within the structure, being therefore determinant for the catalyst activity and efficiency.
To assess the ideal catalyst performance in the catalytic cracking unit, the textural properties are carefully controlled during the catalyst manufacture. Further, to ascertain that the catalyst keeps its optimum condition, following-up these features in the equilibrium catalyst (ecat) with routine sampling is paramount.
The main textural properties of FCCU catalyst include specific area, micropore volume and mesopore area. These properties are commonly assessed with the aid of the nitrogen physisorption technique. Specific area is usually estimated with the aid of the Brunauer, Emmett and Teller method (BET), while the micropore volume and mesopore area are generally determined by means of the T-plot methodology. In this way, it is possible to map in a more precise way the material pore architecture.
Below a summary of the main catalyst textural properties:
Specific Area:
Porous solids have a total surface much higher than that corresponding to the external one because of contribution of pore walls. In the FCCU catalyst, the total surface area is essentially originated from zeolite and alumina. Surface area (SA) is nothing more than the ratio between surface area and catalyst mass.
Technically, AS represents the total area accessible to the adsorbent gases (frequently, N2) by unit of mass (in accordance with the above-mentioned measurement technique). In practice, this variable has usually strong correlation with catalyst activity, because an increase in SA tends to increase active sites availability. That is, probability of contact between hydrocarbons to be cracked and acid sites increases, resulting in higher reaction efficiency.
In the converter, the catalyst is subject to adverse physical chemical conditions that impair its properties, varying in intensity depending on the severity. Extreme hydrothermal conditions, for example, can destroy the zeolite structure, reducing its specific area with time. In spite of the effect of the hydrothermal effect being less accentuated on the matrix specific area, it is affected by the small pores collapse which become larger pores.
A further harmful effect occurs by heavy metals present in crude residual fractions. By contacting the catalyst, they deposit on the particle cavities, altering its properties.
Among the main metal “villains” is vanadium. At elevated feed concentrations and conditions of high temperature and presence of oxygen, this metal tends to form a highly mobile compound (V2O5) which destroys the zeolite crystalline structure by sintering, clogging the acidic sites. Thus, its effect as a contaminant is towards altering both the catalyst selectivity and activity.
To mitigate the deactivation effects on the inventory, it is essential to perform appropriate virgin catalyst make-up, securing the maintenance of acceptable activity levels. The following is an example of a commercial unit which illustrates the correlation of the contaminants content with the specific area loss:
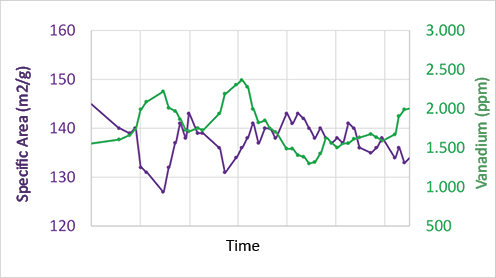
Plot 1- Example of the deleterious effect of vanadium on the catalyst specific area. Source: FCC S.A. database
Pore Volume:
Besides the zeolite intracrystalline cavities, the catalyst particle components include internal gaps of several sizes, which form an extended series of intertwined channels. This amount of particle voids is called pore volume.
The ecat pore volume follow-up can provide hints on the kind of catalyst deactivation faced by the unit. Generally hydrothermal deactivation has a diminished effect on pore volume, in contrast with thermal deactivation which tends to reduce it. It should be emphasized that thermal deactivation is less frequent – occurring under temperature levels above the regenerator normal operation conditions such as flow inversion at TV and continuous torch-oil use.
Pore Diameter:
Pore diameter is the cavities bidimensional opening. Pores should secure that the reagents can reach the active sites and that the products can be removed efficiently. That is, it is essential for the proper diffusion within the catalytic structure. Besides, pore dimension plays a crucial role in selectivity, since the molecules access to their interior is conditioned to their size and shape. For example, a small pore diameter hinders the access of very branched molecules to the internal active centers.
Pore diameters are classed as follows:
- Micropores: < 2 nm (20 Å)
This pore size is to be found only in zeolite. The follow-up of the zeolite content is normally carried out with the aid of the micropore volume (MiPV). Therefore, variations which could occur in MiPV data are directly related to the unit conversion expectations.
- Mesopores: 2 nm a 50 nm (20 Å a 500 Å)
Nearly all mesopores are related to the catalyst active matrix. The mesopores catalyst sites are not as selective as those of the zeolite, however they are able to crack larger molecules which cannot enter the micropores. Therefore, they are the main entities responsible for bottoms conversion. Follow-up is usually by mesopore area (MSA).
- Macropores: > 50 nm (500 Å)
These enable huge molecules present in the feed to penetrate the pores. Based on the nature of the FCC catalyst, a significant amount of macropores in its structure is not expected.
Throughout a catalytic reaction, the presence of macro and mesopores secures both accessibility and efficient reagents and products transport to the pores, while micropores enable the desired selectivity. Thus, together, zeolite and matrix form a synergistic combination that maximizes the catalytic cracking efficiency catalyst
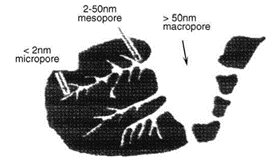
Figure 1- Illustration of pore in a particle. Source: Détermination expérimentale de la distribution de taille de pores d’un milieu poreux par l’injection d’un fluide à seuil ou analyse harmonique. Malvault, G. 2013
Final considerations:
The competency for providing truthful analytical results and specialized technical follow-up is crucial to secure well-grounded, solid decisions. Based on top-ranked methods and following the stringest standards, the FCC S.A. Center for Performance and Development (CPD) is in charge of these and other advanced laboratory analyses. A further differential is the Core Services – a Technical follow-up provided by the expert FCC S.A. engineering team aiming at maximizing customers’ performance.





































What did you make of the publication?