
INTRODUCTION
Located in the distillation unit, desalter plays a crucial role in removing crude oil salts and impurities, protecting equipment and securing efficiency in the complex petroleum refining process. Notwithstanding, its importance goes beyond its function in the distillation unit, affecting also the Fluidized Catalytic Cracking Unit (FCCU). Desalter operational drawbacks can jeopardize crude quality, intervene in FCCU feedstock and contaminate catalyst which can negatively impact production and quality of unit products.
What is the objective of a petroleum desalter?
Desalter (Figure 1) obejctive is to reduce crude oil BS&W (basic sediment and water) and other minerals by water washing. Such removal includes:
- Salts: Chiefly sodium (Na), potassium (K), calcium (Ca) and Magnesium (Mg) chlorides and sulfates;
- Sediments: such as silt (a kind of soil), sand, mud, iron oxide and sulfides;
- Water: Present in soluble form, emulsified and finely dispersed.
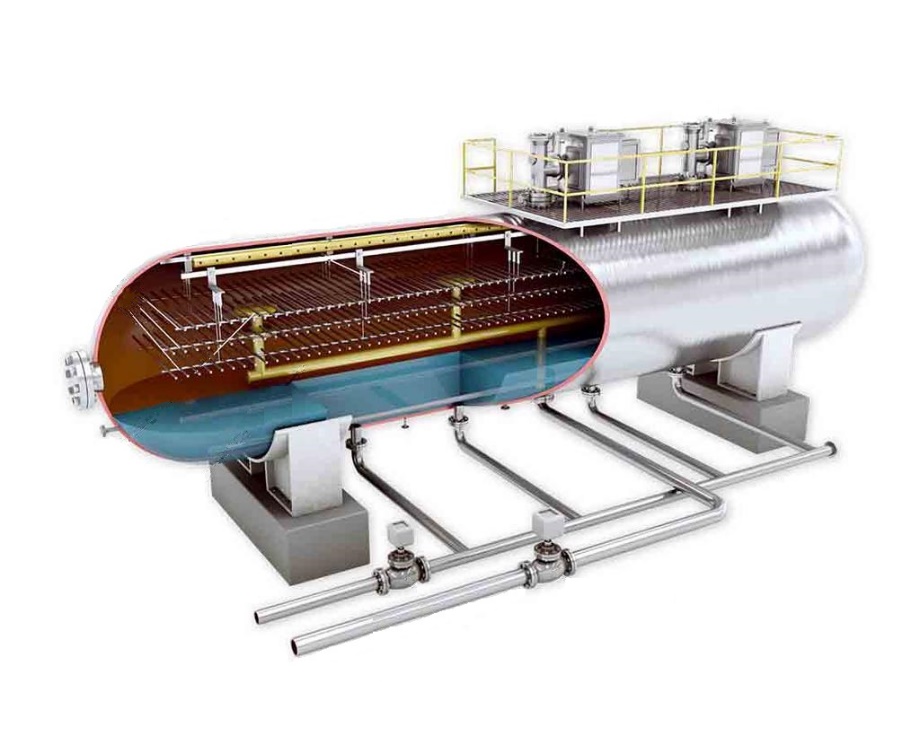
Figure 1: Schematic drawing of the desalting device. Source: modified from RAJ, V.
It is mandatory to remove undesirable impurities from raw crude oil before processing it in the distillation unit because:
- Inorganic impurities such as sodium, calcium and magnesium chlorides can cause scales in areas where water phase changes into vapor (3) (heat exchangers, column trays, etc.) and cause corrosion;
- Hydrochloric acid (HCl) is formed by decomposition of magnesium and calcium chlorides at high temperatures (around 350°C) and leads to equipment corrosion, resulting in lower-than-expected useful life (3);
- Sand or Mud present in crude oil can damage pumps and tubing systems, requiring further maintenance.
Which variables affect desalter efficiency?
A few factors affect the desalter efficiency such as (2):
- Temperature: crude oil and water admixture should be at an optimum temperature to not affect petroleum viscosity and density operational ranges which jeopardize desalting efficiency;
- Washing water: washing water should be injected in sufficient amount to dissolve the salt content (from 4 to 8% v/v) (2);
- Washing water pH value: washing water pH should be kept at 7 to secure efficiency (not affecting water conductivity);
- Water-Crude Interface Level: Such level should be kept constant to avoid entrainment of water to crude or entrainment of crude to water. Emulsions can hinder interface control.
- Mixing Valve Pressure Drop: Accentuated pressure drop yields a stable fine emulsion and consequently, better washing. However, in case of excessive pressure drop emulsion can be hard to break.
- Crude Density: Desalting heavy crudes is a greater challenge for refiners because reduced density difference in aqueous and oil phases renders separation difficult, besides higher content in emulsion-stabilizing compounds in heavier crudes (asphaltenes, for example) (9);
- Demulsifying Chemical Products: demulsifying agent nature affects the efficiency of crude-water separation.
FCCU and catalyst contamination: which relation with the desalter?
Low desalter efficiency in the distillation unit can jeopardize FCCU catalyst performance by introducing more contaminants in feedstock and rise catalyst impurities contents, especially of:
- Sodium;
- Calcium;
- Potassium;
- Magnesium;
- Chlorides
FCCU catalyst contaminants and their effects
Alkaline Metals (Sodium, Calcium, Magnesium, Potassium)
Sodium present in equilibrium catalyst is the sum of feedstock and fresh catalyst sodium:

Fresh catalyst production reveals a minimum amount of sodium easily identified by the manufacturer analyses. However, excess of contaminant sodium from other origins can cause deleterious effects on catalyst:
- Causes the crystalline structure to collapse at lower temperatures (this process is analogous to the use of salt to reduce ice melting temperature) (1) and leads to reduction in specific area;
- It is a potent, irreversible contaminant, which deactivates catalyst by neutralizing acidic sites nearly instantly;
- Zeolite loss of activity means lower conversions, and higher bottoms production, besides loss in naphtha octane (1);
- Shows synergy with vanadium, together they can increase zeolite destruction at a higher rate than when acting separately;
Sodium-vanadium interaction – besides steam and high temperature of FCCU regenerator – leads to low melting point compounds, as can be seen in Table 1, which makes vanadium mobility easier and contributes for zeolite crystalline structure collapse.
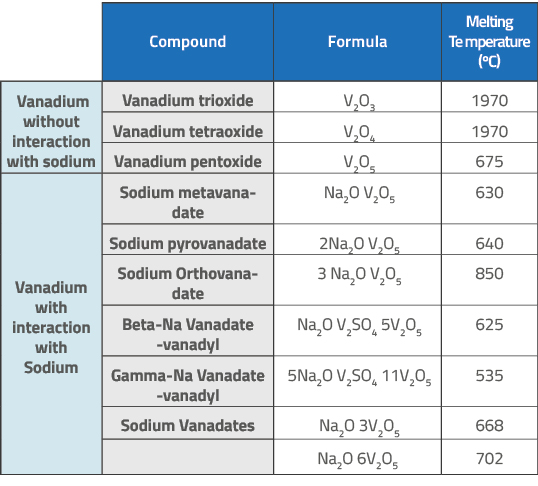
Table 1: Catalyst contaminants in the presence of sodium and their melting points ((ELIZA, D. Análises de ecat,2023).
Calcium contamination causes FCCU catalyst deactivation by neutralizing acidic sites, analogously to sodium, and hydrothermal stability reduction leads to zeolite structure collapse. (5)
Magnesium acts in a less aggressive way than sodium to reduce activity. Potassium poisoning is rare and its effect is also similar to that of sodium, but to a lesser extent (1).
Chlorides
In spite of the fact that they do not deposit and do not affect the catalyst itself, chlorides have two main deleterious effects for the FCCU:
- Catalyst: the presence of chlorides can reactivate catalyst nickel species already passivated increasing coke and hydrogen yields in the unit (4);
- Equipment corrosion: chlorides are transformed into hydrochloric acid (HCl) when they reach the riser which tends to form NH4Cl (ammonium chloride) in the fractionator upper system. NH4Cl deposits on trays and heat exchangers (6) causing scales and corrosion.
The action of these contaminants reinforces the importance of continuously monitoring the amount of feedstock and e-cat by means of frequent analyses, since they cause harmful effects to the FCCU as a whole.
Bibliography:
- LETZSCH, Warren S. Deactivation of FCC catalysts. Digital Refining, jul. 2022. Available at: https://www.digitalrefining.com/article/1003121/deactivation-of-fcc-catalysts. Access on: July 25, 2024.
- RAJ, Vaibhav. Crude Oil desalter: Purpose, Working, Types, Parts, variables. All about piping, Mechanical Engineering. Available at:https://www.allaboutpiping.com/what-is-desalter/. Access on: July 26, 2024.
- PEREIRA, J. et al. Crude Oil Desalting Process. Advances in Petrochemicals. InTech, 2015. Available at:http://dx.doi.org/10.5772/61274. Access on: July 26, 2024.
- SENTER, Corbett, et al. Role of chlorides in reactivation of contaminant nickel on fluid catalytic cracking (FCC) catalysts. Applied Catalysis A: General, Volume 611,2021. Available at:https://www.sciencedirect.com/science/article/pii/S0926860X20305718. Access on:July 30, 2024.
- BAI, Peng et al. Fluid catalytic cracking technology: current status and recent discoveries on catalyst contamination. Catalysis Reviews 61 (2018): 333 - 405. Available at:https://www.tandfonline.com/doi/full/10.1080/01614940.2018.1549011. Access on: July 30, 2024.
- DIAMANTE, Eliza. Integração de Unidades de Refino com o FCC: Destilação e Hidrodessulfurização de Naftas. FCC S.A. Artigo, 2024. Available at:https://www.fccsa.com.br/pt/fcc-connect/todas-publicacoes/integracao-de-unidades-de-refino-com-o-fcc-destila/. Access on July 30, 2024.
- BERNOIT, B and ZURLO, J. Overcoming the challenges of tight/shale oil refining. Digital Refining, abr. 2014. Available at:https://www.digitalrefining.com/article/1000979/overcoming-the-challenges-of-tight-shale-oil-refining. Access on:July 31, 2024
- MEIRA, A. S. e LUCENA, S. C. Características e Aspectos Econômicos do Refino de Petróleo. IBP – Instituto Brasileiro de Petróleo, Gás e Biocombustíveis, mar. 2014.
- SILVA, M. W. da S. A short guide of problem solving for crude oil refining processes. Linkedin Publication, out. 2023. Available at:https://www.linkedin.com/pulse/short-guide-problem-solving-crude-oil-refining-da-silva-mba--qlfyf. Access on:July 31, 2014
- DIAMANTE, Eliza. Análises de e-cat. Material interno FCC S.A., 2023.

































What did you make of the publication?