Introduction
The presence of contaminating metals in FCC loads represents a recurring challenge for refiners, especially in the case of elements such as nickel, vanadium, sodium and iron. Although catalyst providers have developed solutions over the years to handle Ni and V, such as specific catalytic formulations, passivating additives and operational strategies, the approaches available for controlling Fe are still not very effective. The operational response often comes down to increasing catalyst replacement or adding flushing, which affects the profitability of the refinery and compromises the FCC unit performance. Furthermore, with the increasing use of heavier loads, such as tight oils, the deleterious effects of iron contamination have become more recurrent and intense in UFCC operations [1-4].
Unlike vanadium, iron does not deactivate the catalyst through dealuminization reactions. Instead, Fe tends to deposit on the outer surface of the catalytic particles, forming nodules rich in iron, silicon, calcium and sodium that block access to the active sites and impair the fluidization of the catalyst [5-7]. As iron accumulates in the equilibrium catalyst (Ecat), refiners can see significant reductions in accessibility (a measure that estimates how easily the load diffuses into the particle), apparent density (ABD) and, consequently, in the conversion and cracking efficiency of bottoms. In certain cases, high iron levels are also associated with increased SOx emissions and higher temperatures in the regenerator.
Despite these operational impacts, the mechanisms of iron deactivation have been less studied than those caused by other contaminants, which has limited the development of effective catalytic solutions [8,9]. Recognizing this challenge, FCC S.A., in partnership with Petrobras and Ketjen, has launched a long-term R&D initiative aiming to better understand the interaction between iron and FCC catalytic components, as well as studying materials capable of resisting the effects caused by this contaminant.
This article presents the main results of this initiative, highlighting laboratory and commercial data that help clarify the mechanisms of iron-induced catalytic deactivation. The SaFeGuard™ technology was also introduced, already validated both in laboratory tests and in commercial FCC units. This is a new generation of catalysts with high resistance to iron contamination, developed specifically to operate in environments with high levels of this metal, while maintaining the catalytic performance and operational reliability of the unit.
1. Effect of Fe on Ecat properties
As iron contamination in FCC loads increases, its effects on the equilibrium catalyst (Ecat) become more evident and damaging. As already explained, iron tends to accumulate on the outer surface of catalytic particles, blocking pores. These changes in catalyst morphology reduce accessibility to acid sites, compromise fluidization properties and increase friction between particles, intensifying the rate of catalyst wear [7-10].
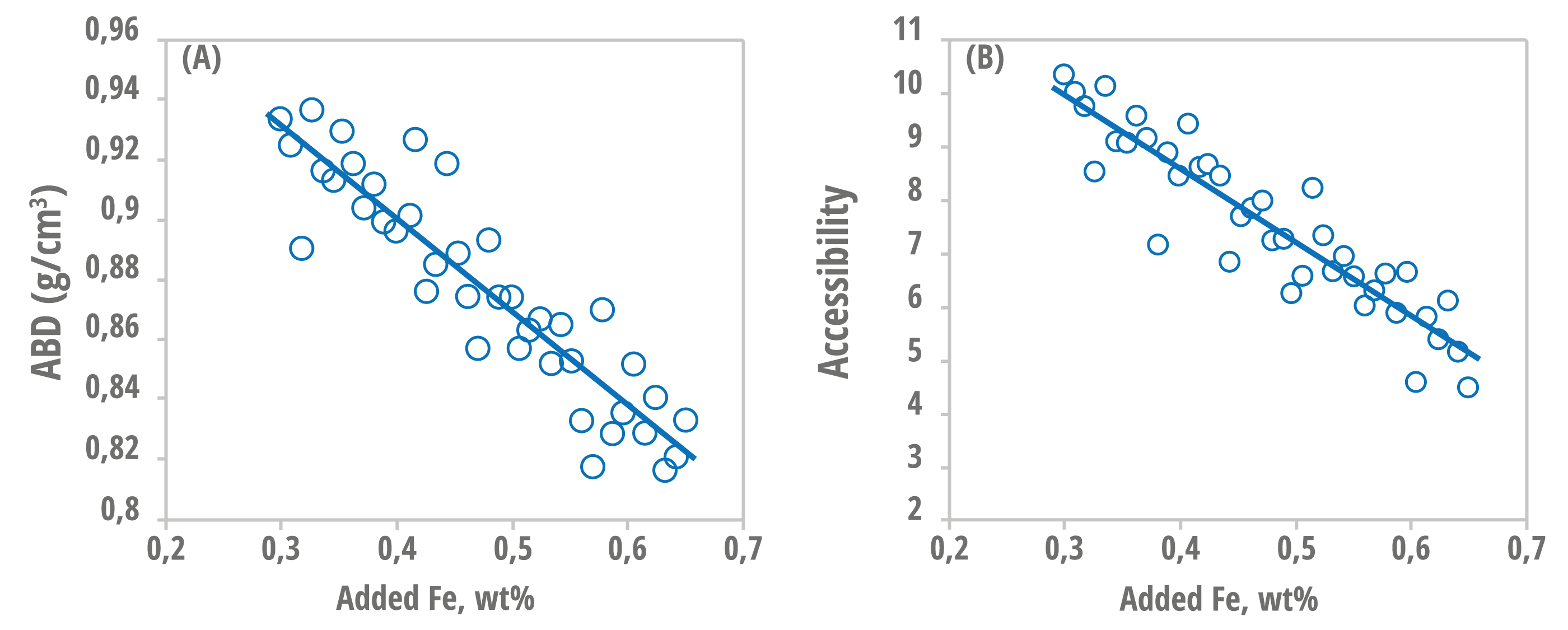
Two critical properties commonly affected by the presence of Fe are bulk density and accessibility. Figure 1 shows a clear correlation (R² > 0.85 in both cases) between the increase in iron content and the decrease in density and accessibility. Catalysts with a high Fe content show a significant drop in accessibility (Figure 1B), which can hinder contact between the heavier molecules in the load and the active sites. At the same time, there is a reduction in density (Figure 1A), which can have a negative impact on the fluidization behavior and even circulation of the catalyst in the unit.
Figure 1 – (A) Correlation between the added Fe content (% by mass) and the apparent catalyst density (g/cm³);
(B) Correlation between the added Fe content (% by mass) and the catalyst accessibility. Figure adapted from [7].
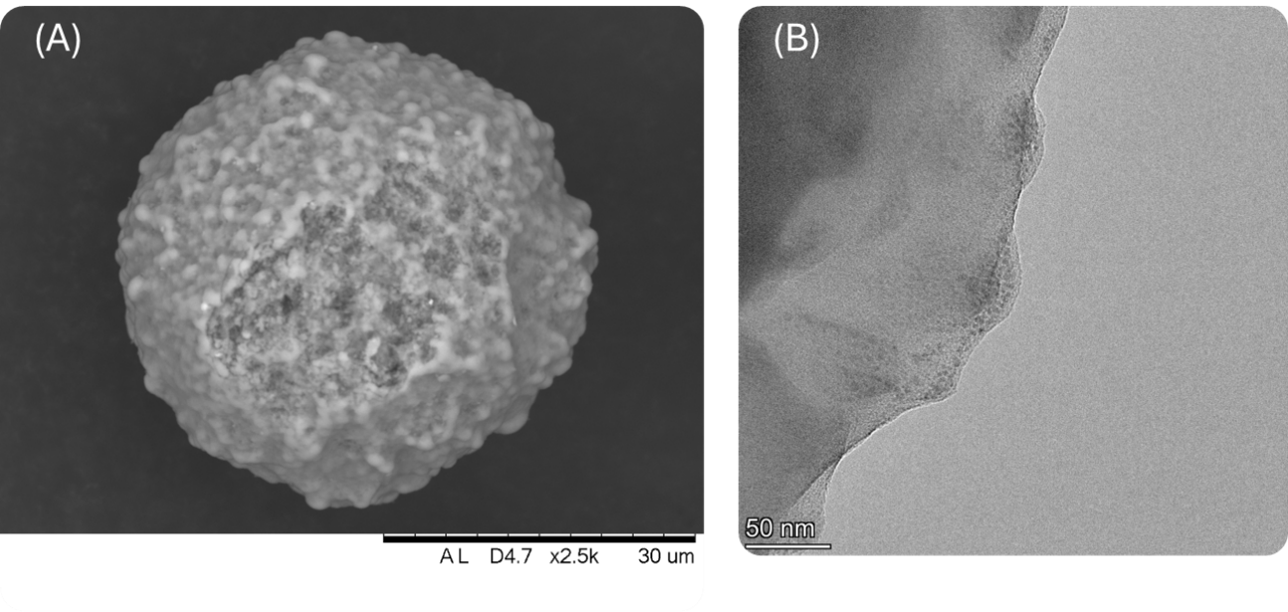
Scanning and transmission microscopic analyses, SEM and MET, provide additional insight into the impact of iron on catalyst morphology. Figure 2 shows the morphological characteristics of an Ecat with a high Fe content. In Figure 2A, the micrograph obtained by scanning electron microscopy (SEM) reveals the formation of nodules on the surface, which are absent in equilibrium catalysts with low iron content. Figure 2B, obtained by transmission electron microscopy (TEM), shows in greater detail the shape of these nodules at the edge of the particle on a nanometric scale (50 nm).
These morphological changes have a direct impact on the catalyst's performance, resulting in lower conversion, poorer yields of the products of interest, such as lower gasoline production, greater coke formation and increased bottoms. These variations are sometimes mistakenly attributed to the quality of the load or the presence of other metals, but advanced diagnostics can confirm typical iron-related deactivation patterns. Therefore, accurately identifying and mitigating the effects of Fe is essential to preserving the profitability of the FCC unit.
Figure 2 – (A) Scanning Electron Microscopy of an Ecat particle contaminated with high Fe content.
(B) Transmission Electron Microscopy of the particle edge of an Ecat contaminated with high Fe content. Figure adapted from [7].
2. Development of specific technology for Fe contamination
One of the main challenges in developing iron-resistant FCC catalysts is to accurately reproduce, under laboratory conditions, the deactivation effects induced by this contaminant on a commercial scale [10]. Traditional laboratory deactivation protocols, such as Mitchel impregnation followed by hydrothermal deactivation, ANCD-4 or CPS, fail to replicate the textural and surface transformations characteristic of the iron presence, particularly the formation of nodules (Figures 2A and 2B), the reduction in accessibility and density (see Figure 1A and 1B).
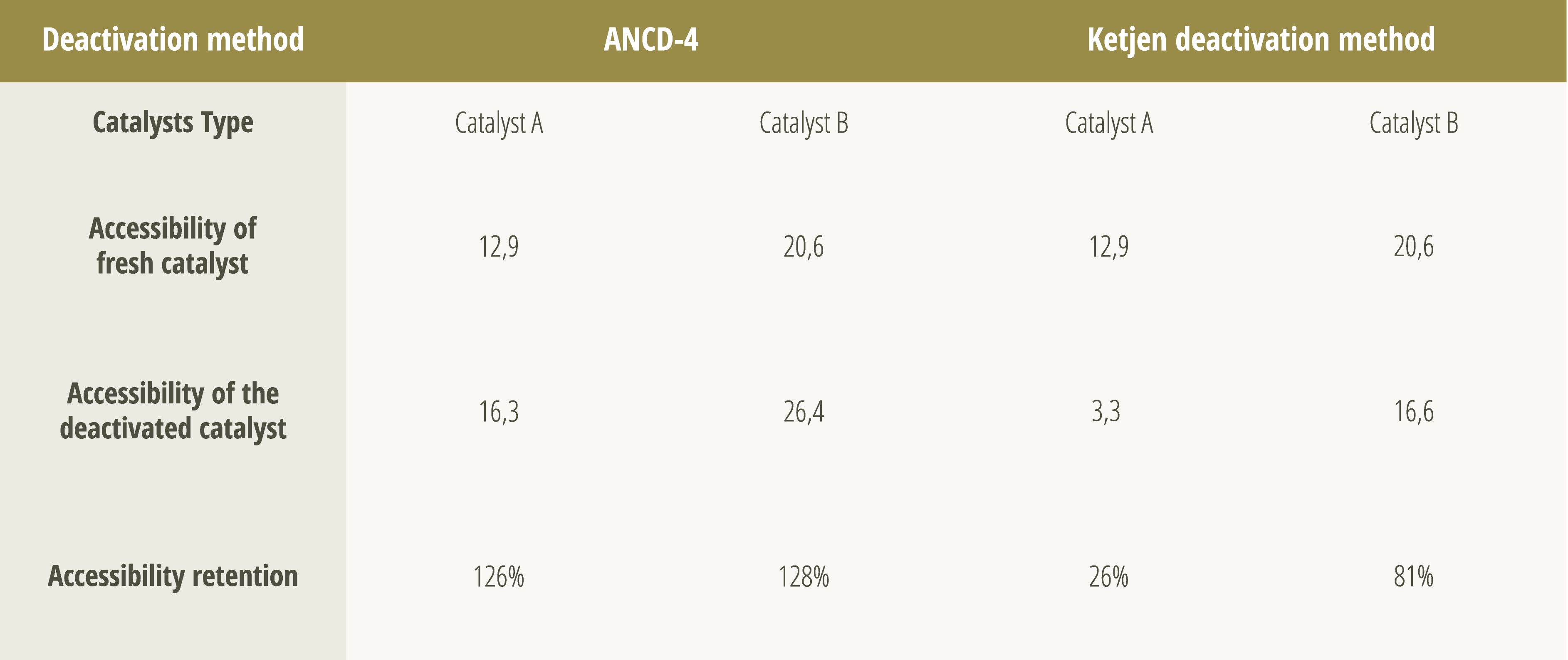
To fill this gap, Ketjen, in partnership with Petrobras and FCC S.A., has developed a deactivation protocol capable of simulating the effects of iron contamination in a more realistic and representative way. As shown in Table 1, this approach was effective in differentiating catalytic technologies in terms of accessibility retention after deactivation with Fe. While traditional protocols such as ANCD-4 showed an artificial increase in accessibility, Ketjen's protocol more faithfully reproduced the behavior observed in commercial FCC units, with a progressive drop in accessibility as iron accumulates, in line with equilibrium catalyst data. The development of this method allows to assess, in a simple and systematic way, which technologies are most resistant to iron contamination.
Table 1 – Accessibility and accessibility retention of two different catalytic technologies after deactivation
in a laboratory containing Fe, using the ANCD-4 and Ketjen protocols. Table adapted from [6].
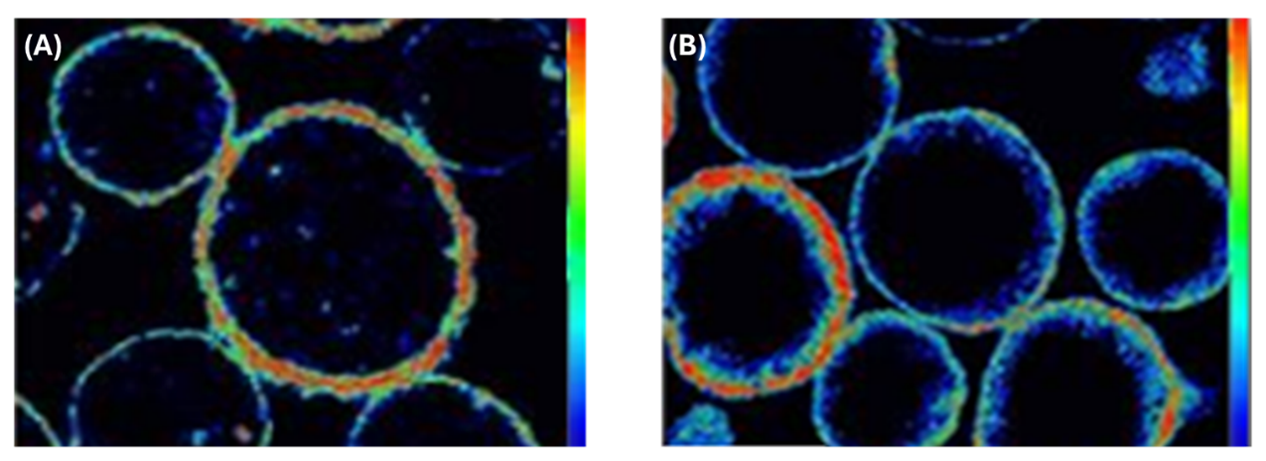
Further validation of the Ketjen deactivation methodology is shown in Figure 3. Image 3A shows the location of Fe in an Ecat with high iron contamination. This figure shows that Fe is predominantly deposited on the edges of the particles, corroborating Figure 2A and 2 B. The red color indicates a high concentration of iron.
Moreover, image 3B shows a catalyst artificially deactivated in the laboratory using the protocol developed by Ketjen. Notably, the distribution of iron in this sample faithfully reproduces the pattern observed in commercial Ecat (Figure 3A), with Fe mostly concentrated on the outer surface of the particle. This result is particularly relevant since, to the best of our knowledge, no other catalyst supplier has so far demonstrated the ability to replicate under controlled laboratory conditions such a realistic pattern of iron deposition on the catalyst on a laboratory scale.
Figure 3 – (A) Location and distribution of iron in commercial Ecat.
(B) Location and distribution of iron in the deactivated catalyst in the laboratory using Ketjen's protocol. Figure adapted from [6].
These advances are fundamental to the development of the next generation of iron-resistant catalysts. By creating decommissioning conditions that more realistically mimic the iron poisoning observed in operation, Ketjen and FCC S.A. can evaluate different technologies and formulations more accurately, identifying more effective solutions. This approach allows for the rational design of materials such as SaFeGuard™, designed to preserve the porosity of the catalyst while minimizing iron-induced deactivation mechanisms. This is a significant step forward for refineries facing increasing Fe contamination in their operations.
It is worth mentioning that FCC S.A. already has catalytic solutions with proven iron tolerance, such as the Upgrader™ and Denali™ catalysts, both developed with a focus on high accessibility and optimized pore distribution. These structural characteristics mean that the material can tolerate significant levels of iron contamination without prematurely reaching critical accessibility, the point at which the catalyst's performance begins to compromise the refinery's operations and profitability.
The differential of SaFeGuard™ lies in its advanced catalytic formulation, which combines high accessibility with a modification to the catalyst matrix system capable of reducing the vitrification reactions that occur on the catalyst surface in the presence of iron. As a result, SaFeGuard™ has better accessibility retention than other technologies on the market, and is especially suitable for refineries that operate with extremely high levels of this contaminant.
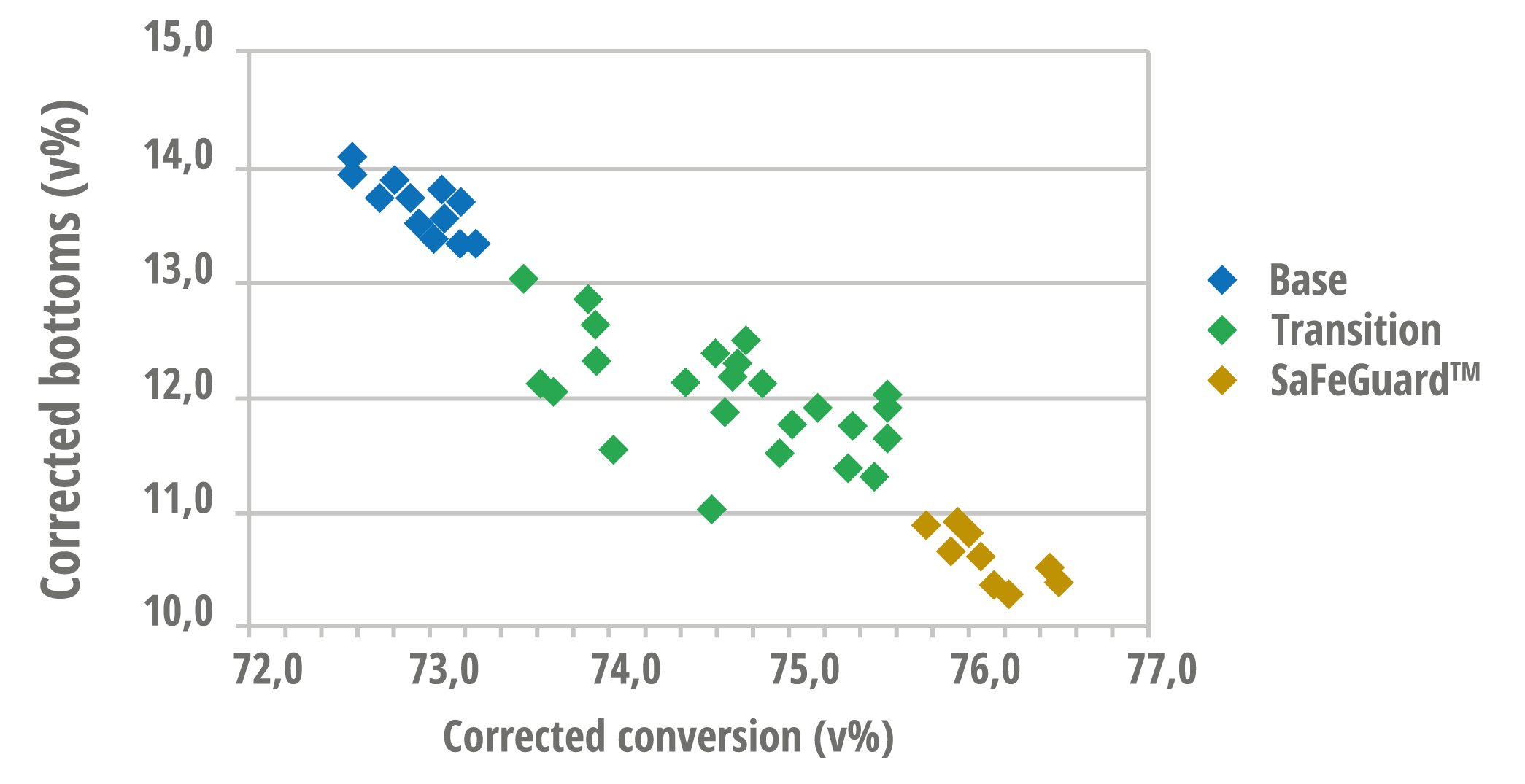
Figure 4 reinforces these benefits by comparing SaFeGuard™ with a highly accessible FCC S.A. base catalyst, where it can be seen that SaFeGuard™ performed better than the base catalyst, with lower yields of bottoms and higher conversions. Figure 4 shows that it is possible to see conversion gains as early as the inventory transition, even under high iron contamination conditions (~5000 ppm Fe). This happens since the accessibility of SaFeGuard™ is more preserved than that of the base catalyst, which maintains the technology's ability to convert more bottoms, even under severe impact from metals such as iron.
Figure 4 – Relationship between conversion and production of bottoms for different catalytic technologies
with high accessibility, under conditions of high iron content (~5000 ppm). Figure adapted from [6].
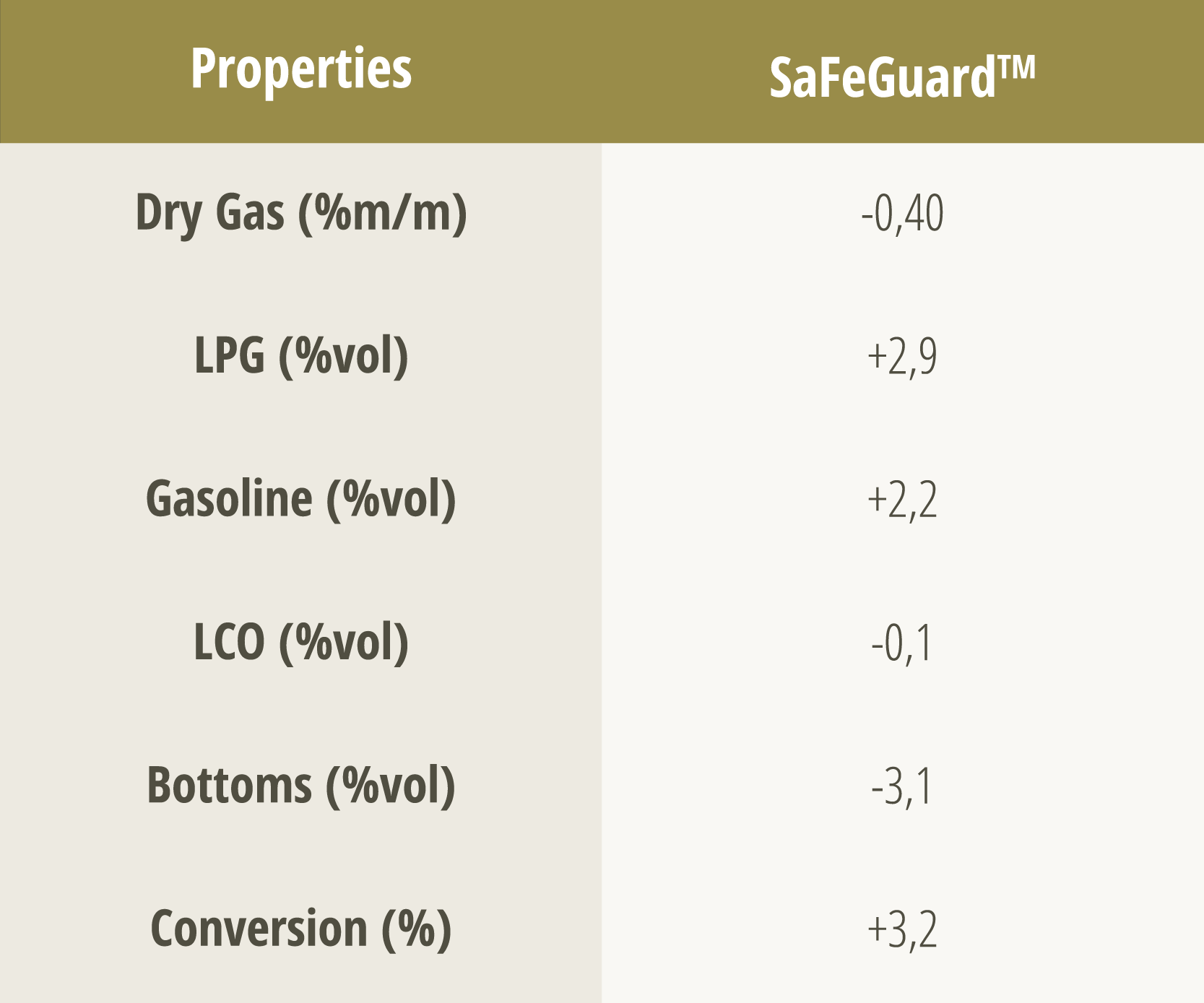
Tables 2 and 3 show the main results of the commercial evaluation in an FCC unit, comparing SaFeGuard™ technology to the base catalyst under conditions of high levels of metal contaminants. Even with higher levels of iron (0.56% vs. 0.48%) and sodium (0.26% vs. 0.18%), SaFeGuard™ showed superior performance, evidenced mainly by its greater accessibility. This factor contributed to a significant increase in conversion, coupled with a significant reduction in bottoms formation and an increase in gasoline yields.
Furthermore, the replacement rate of the SaFeGuard™ catalyst was lower than that of the base catalyst (0.44 vs. 0.48 lb/bbl), indicating not only greater catalytic performance, but also the potential to reduce operating costs. These results prove that the SaFeGuard™ formulation provides greater resistance to metal deactivation and, consequently, greater selectivity for high value-added products, even under severe operating conditions.
Table 2 – Comparison of the properties of different technologies in an industrial test. Table adapted from [6].
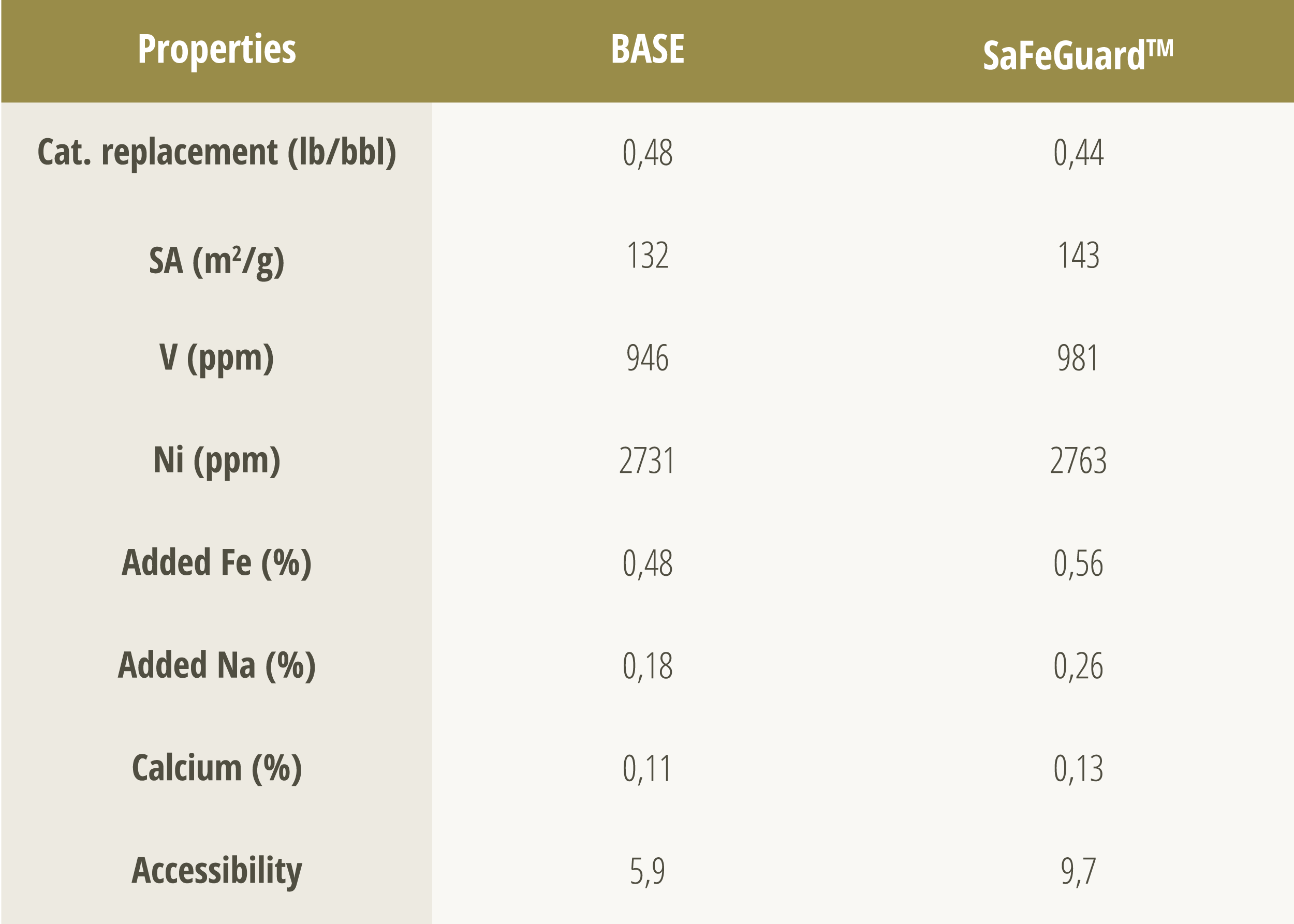
Table 3 – Variation in product yield when using SaFeGuardTM in a commercial unit. Table adapted from [6].
SaFeGuard™ is a paradigm shift in the refining industry, especially in units operating in highly iron-contaminated environments. It has been designed to maintain excellent activity and conversion of bottoms even in extreme conditions, guaranteeing technical performance and profitability, even in the face of sudden fluctuations in load quality.
Faced with the growing adoption of challenging loads with high levels of metal contaminants, SaFeGuard™ has emerged as a strategic technological solution. Its formulation protects against chemical and morphological changes while maintaining catalytic performance and operational stability. With SaFeGuard™, the refiner can safely process more complex cargoes and explore new opportunities on the refining horizon.





































What did you make of the publication?