The similarities between the indices are considerable since both assess fuels properties utilizing hydrocarbon molecules as reference. A further interesting similarity of these indices is that their measurement experiments employ engines to quantify their value (ASTM 2700, ASTM 2699, ASTM D-613). The similarities however end at this point.
Whereas Octane represents a certain fuel resistance to detonation, Cetane Number measures the explosiveness (or spontaneous combustion) of a fuel when exposed to heat and suitable pressures. Such difference is linked directly to the kind of engine in which the fuel will be used. Gasoline is normally used for Otto Cycle engines, where admission is made with the mixture of air and fuel, while Diesel fuel is directed to engines where the admission phase is made with air only and fuel is injected just at the final compression section of the engine pistons, also called diesel cycle.
A further difference would be the scales of each of these indices. While octane sets forth an equivalence relationship of the percentage of a mixture of isooctane (2,2,4-trimethylpentane) and n-heptane, as can be seen in Figure 1, the Cetane number provides a relationship between n-cetane (Hexadecane) and heptamethylnonane of reference value 15 as indicated in Figure 2.
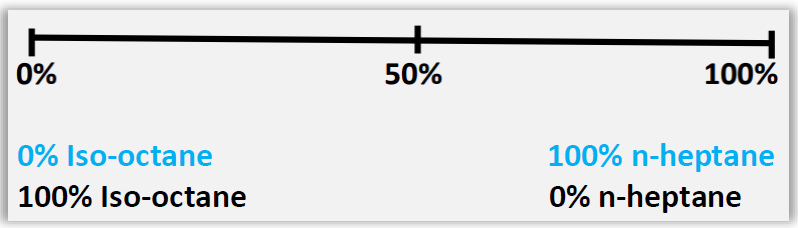
Figure 1 – Octane Scale
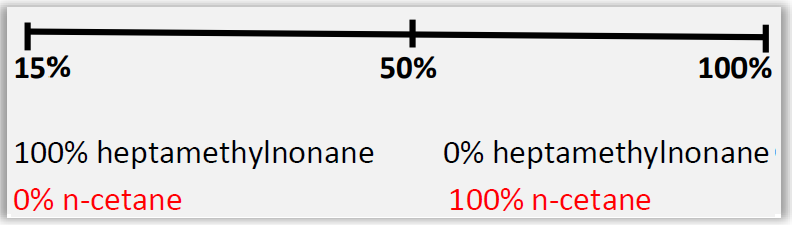
Figure 2 – Cetane Number Scale
Upon examining the reference scales of each index, the conflicting character between compressibility and explosiveness becomes further clearer by observing the reference molecules structures as indicated in Figure 3.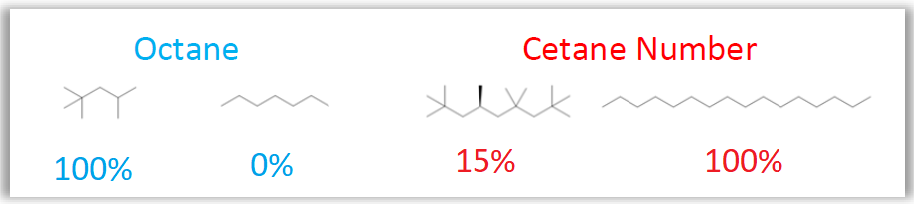
Figure 3 – Structure of the indices reference molecules
Based on this information, it is evident that trends that favor the response of a molecule in one index worsen its performance in the other one. For example, while a raise in unsaturation, aromaticity and branching worsen cetane number, these same features increase molecules octane.
This can be observed in a very clear way on the previous reference molecule for 0% cetane number,  - methylnaphthalene, the molecular structure of which is represented in Figure 4.
- methylnaphthalene, the molecular structure of which is represented in Figure 4.

Figure 4 –  -methylnaphthalene molecular structure
-methylnaphthalene molecular structure
Therefore, to obtain a high Cetane Number stream, it is advisable to look for poorly unsaturated, scarcely branched, high-molecular weight molecules. That is, ideally it would mean paraffins having viscosity suitable for injection in engines.
To heighten the cetane number of a stream various arrangements can be made including favor hydrogen transfer or hydrogenation reactions, alter the fractioning cut-off, adjust the operation of the treating unit, etc. However these adjustments should be evaluated in line with the commercialization team since this could lead to significant cost increases or open new possibilities for monetizing a unit.
In case you need any further information on this matter, please contact the FCC .S.A. team to aid the decision-making.
-

- de
-


































What did you make of the publication?