Automotive Gasoline is the most common fuel for light duty vehicles equipped with Otto Cycle engines.
In the Otto Cycle fuel is compressed with air for combustion and to have a fair performance the fuel should resist rises in pressure and temperature created during the compression phase of the cycle without getting into self-combustion, since fuel is compressed with air – this is known as anti-knock feature.
The measurement of the gasoline anti-knock feature is known as Octane. The test is run on special engines where the fuel compression rate is compared with that of standards, the compression rate being varied until attaining a standard knock intensity which is electronically measured.
The standards are mixtures with known volumetric fractions of isooctane – 2,2,4-trimethyl pentane (Octane 100) and n-heptane (Octane 0). The octane number represents the volumetric percentage of isooctane in a mixture with n-heptane which would exhibit the same anti-knock feature of the tested fuel.
Naphtha octane depends on the hydrocarbon species present. Generally, it varies with the hydrocarbon types in the following order:

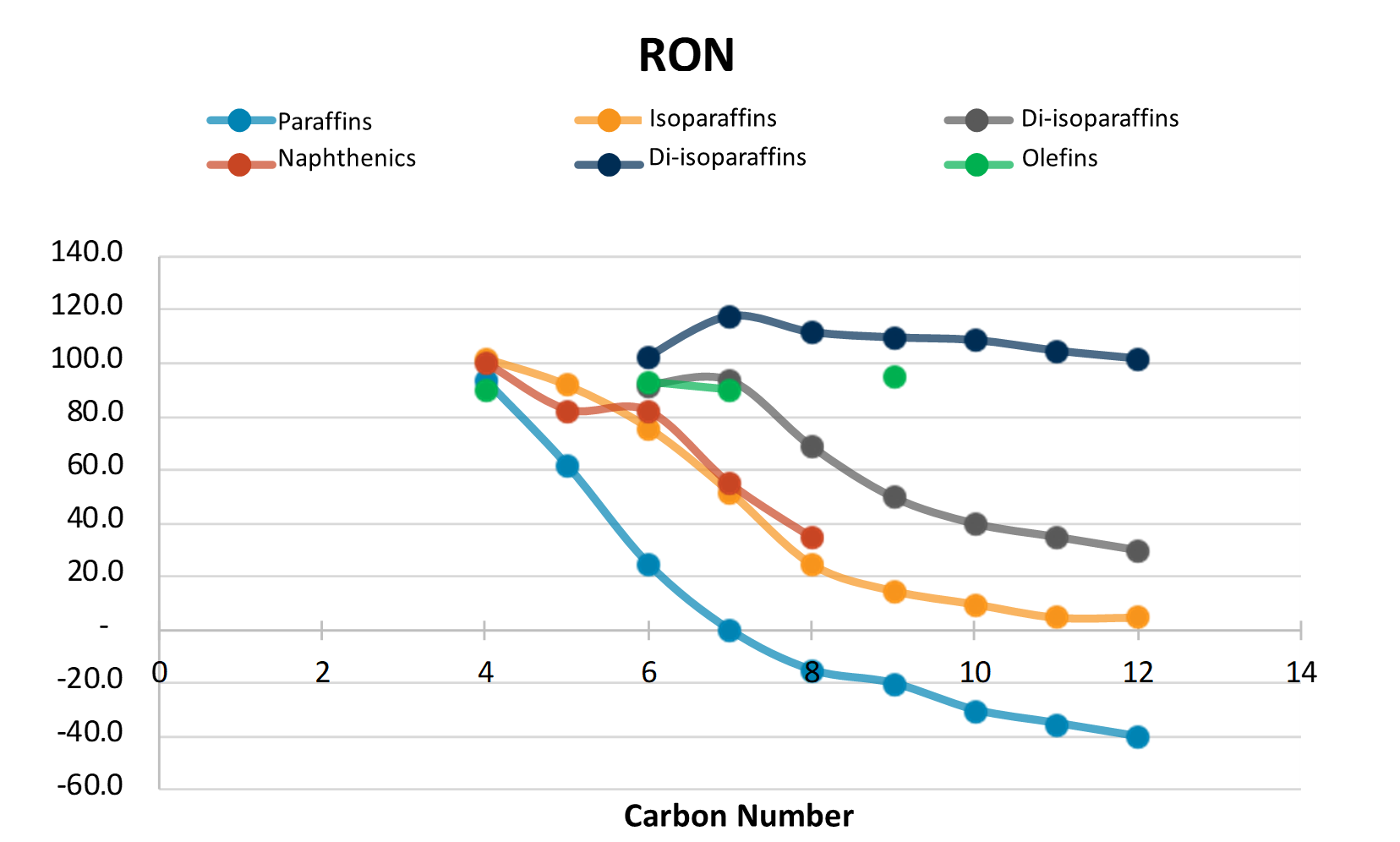
Figure 1 - Octane RON as a function of carbon number and hydrocarbon type
Each naphtha stream making gasoline has a different composition and, therefore, a different octane, which influences its percent in the gasoline mixture:
- Straight distillation naphtha – low octane;
- Catalytic Cracking Naphtha – low octane;
- Delayed Coking Naphtha – medium octane;
- Catalytic reform, isomerization or alkylation naphtha – very high octane.
Knowing the octane of the streams making the mixture is relevant to prepare gasoline mixtures, assess the units and identify the reasons for variations in each gasoline component. The selection of favorable petroleum indexes, rich in naphthenic compounds, for example, can also aid in the octane rise of intermediate products.
Cracked Naphtha:
Generally, this is the main component of the gasoline mixture in a refinery, being responsible for most of the volume. Its octane is usually the limiting factor for the incorporation of other streams into the final gasoline and this is paramount for the refinery profitability, since usually lower octane streams which cannot be incorporated into gasoline have low market value.
Increasing cracked naphtha octane means to perform modifications that concentrate high-octane compounds in this stream, such as aromatics and branched compounds, reducing the amount of paraffins. This can be done by stimulating the cracking of naphtha range paraffins, making them to become LPG, and adjusting the hydrogen transfer reactions to rise branched compounds production.
The adjustment of the final cracked naphtha boiling point can also aid in octane rising, since by rising the higher-than 150°C boiling point naphtha fraction increases high octane products concentration.
Some refineries operate with low final boiling points for light cracked naphtha to adjust sulfur in the gasoline mixture. In this case it is important to invest in the quality of fractioning to prevent LCO boiling range sulfur compounds to reach the light naphtha. High top reflux and heavy cracked naphtha ratios will aid in the adjustment, as well as the improvement in gas-liquid contact at the fractionator top area. Units that operate at low top temperatures and undergo ammonium salts formation in this area normally have fractioning issues between light naphtha and LCO, increasing naphtha sulfur.
For obtaining cracked naphtha rise the following adjustments can be made:
- Rise in the reaction temperature;
- Rise in the final naphtha boiling point;
- Rise in the catalytic activity by increasing specific make-up;
- Use of additives for octane rising;
- Change in the catalyst system formulation for modulation of selectivity and hydrogen transfer reactions;
- Use of chlorine-free catalysts to avoid the worsening of sludges on top of the main fractionator.
The FCC S.A. technical team is available to aid its customers in the optimization of their units.




































What did you make of the publication?